|
|
1.IntroductionDurable broadband silver (Ag) mirrors have long been a goal for astronomical telescopes. Silver’s higher reflectivity and lower emissivity in the thermal infrared spectrum have significant performance benefits over the standard aluminum mirrors currently in widespread use. There have been some qualified successes for silver coatings, notably those used on the Gemini telescopes,1–3 but the fact that so few ground-based telescopes have Ag-based mirrors indicates the elusiveness of the efforts. Many coatings that seem to hold up in the laboratory do not endure well when exposed to an actual observatory environment. Even the successful Gemini coating comes at a cost of sacrificing the deep blue and UV portions of the spectrum which is an unacceptable compromise for many astronomical research programs. The University of California Observatories (UCO) has undertaken a program to develop and/or identify high-performance coatings useful for astronomical optics.4–7 A strong motivation for our research is to develop durable Ag mirror coatings that meet the requirements of the Thirty-Meter Telescope project, which requires high reflectivity from . Although Ag thin films are routinely deposited in various applications, bare Ag quickly tarnishes due to reactions with oxygen and especially sulfur, and it corrodes easily via salt formation with halides. Thus, to provide long-lasting mirrors, Ag must be protected by barrier layers of transparent dielectrics in order to prevent tarnish and corrosion. The design of barrier layers has several constraints, and identifying suitable materials and deposition processes has proven challenging. Phillips et al.4–7 discuss some details on the constraints and challenges and also report that certain materials, notably in combination with high-index oxides, seem promising as barrier layers for Ag surfaces (i.e., barrier overlayers). Telescope mirrors and barrier overlayers have traditionally been coated by physical vapor deposition (PVD). Barrie et al.8 and Chu et al.9 have demonstrated several successful recipes for protected-Ag mirrors using various PVD techniques, and Jobst et al.10 utilized similar deposition methods to deposit aluminum oxide and silicon dioxide as barrier layers for Ag mirrors. However, due to extrinsic factors such as the large areas of telescope mirrors, noncleanroom environment during substrate cleaning, and subsequent film deposition, pinholes are unavoidable and can significantly degrade the coating lifetime by providing moisture and other chemicals a means to permeate through the mirror “stacks” (i.e., a structure made of a Ag mirror layer covered with barrier layers) and cause corrosion. Since PVD is assumed to be line-of-sight deposition, pinholes are exacerbated via self-shadowing. Atomic layer deposition (ALD), being conformal over complex three-dimensional structures, holds the promise of pinhole-free barrier layers. Although ALD is a defined subset of chemical vapor deposition techniques, it is specifically advantageous for deposition processes requiring low temperatures, especially when the reactant gas is energized in a plasma, a process known as plasma-enhanced atomic layer deposition (PEALD). Furthermore, the low-stress, amorphous nature of ALD films is expected to improve overall mechanical durability as well by reducing or eliminating weak microcrystalline grain boundaries. The excellent thickness uniformity achievable with ALD is another benefit. Here, we report results of a pilot study to investigate whether the ALD process can be used to improve the durability of Ag mirror stacks for astronomical telescopes. 2.Sample Preparation and Experimental DetailsBriefly, mirror samples were prepared with most layers deposited by PVD in the UCO coating chamber. The layers were produced either with direct electron beam (e-beam) deposition, or reactively with e-beam deposition under ion bombardment. The final top layer was added either with PVD or ALD to provide a direct comparison in performance. The two coating “recipes” are shown in Figs. 1 and 2. PVD deposition of all thin film layers except the top barrier layers were done simultaneously for samples A1 and A2 and again simultaneously in a another run for samples B1 and B2 to ensure that the only variable is the top layer. This allows an accurate comparison of the PVD versus ALD top-layer performance. The samples were characterized for reflectivity and then subjected to severe environmental stressing, roughly 10 days at 80°C and 80% relative humidity. Fig. 1Schematic of samples A1 and A2 showing experimentally deposited layers. Note that the 3 nm antioxidation layer deposited from e-beamed protects the Ag from oxygen ions during the reactive deposition of the atomic layer deposition or physical vapor deposition layer. 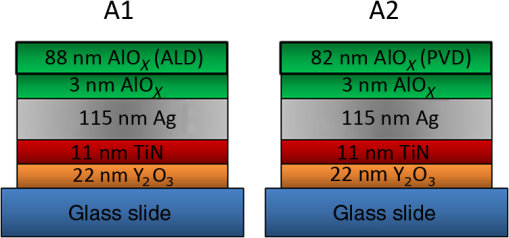 Fig. 2Schematic of samples B1 and B2 showing experimentally deposited layers. In this case, acts as the antioxidation which protects the Ag. This stack has enhanced reflectivity in the blue/UV. 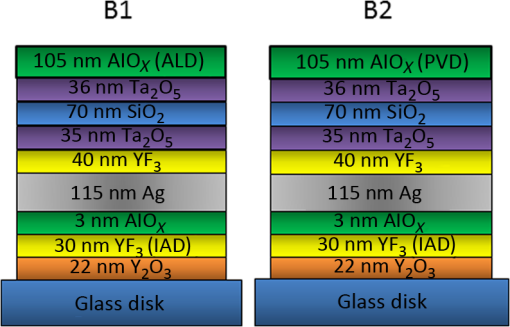 Mirror samples A1 and A2 were prepared on glass microscope slides, and samples B1 and B2 were prepared on 2-in. diameter BK7 glass disks. Most thin film layers, including Ag layers, were deposited by conventional e-beam evaporation in the custom PVD chamber. These depositions were done at room temperature and base pressure of , and the growth rate was calibrated using a quartz crystal microbalance within the chamber. All four samples contain a single 115 nm Ag layer sandwiched by underlayers (i.e., layers formed between the substrate and Ag layer) and overlayers (i.e., layers formed on top of the Ag layer). The layers in samples B1 and B2 were deposited using ion-assisted deposition (IAD) in an inert argon gas for densification and are designated in Fig. 2 by IAD in the illustration. Ion assist is also used with oxygen introduced to the chamber to deposit an oxide reactively. While both processes are considered to be reactive e-beam IAD, we focus observations on the top oxide layers and will, therefore, use the term “reactive e-beam IAD” to refer to IAD in an oxygen environment. The overlayers were coated with a single top barrier layer deposited by either reactive e-beam IAD or PEALD. Samples A2 and B2 similarly received a top aluminum oxide layer with reactive e-beam IAD. Samples A1 and B1 had their final layers deposited using PEALD. Prior to PEALD deposition, the PVD-produced mirrors were rinsed using organic solvents in a clean room environment. Varying thicknesses of aluminum oxide () were deposited using an Oxford FlexAl ALD tool. Trimethylaluminum and oxygen gas ionized by inductively coupled plasma were used as aluminum and oxygen reactant sources, respectively. Substrate temperature and pressure were held at 150°C and 25 mTorr, respectively, and plasma RF power was constant at 300 W during oxygen gas ionization. Growth rate was calibrated using spectroscopic ellipsometry that provided both thickness and optical constants. During all sample preparation steps, vacuum was broken only to transfer samples from the custom PVD chamber to the PEALD chamber. The samples were measured for reflectivity on a Varian-Cary 5000 spectrophotometer to ensure that the experimentally obtained spectrum for as-prepared specific mirror stacks closely matched that of the ideally designed mirror stacks. Samples A1 and A2 shown in Fig. 1 demonstrate the initial direct comparison between reactive e-beam IAD and PEALD barrier layers. Both samples utilized e-beam deposition in the PVD chamber to deposit identical films on glass slides up to the 3-nm layer. This coating recipe, along with all samples tested in this experiment, utilizes 22 nm as a stripping layer on glass substrates to aid in the recoating process of mirror coating recipes applied to reusable optics.11 The underlayer of 11 nm TiN serves as the intermediate adhesion layer prior to Ag deposition due to the tested success of TiN as a base layer for smooth metal film deposition.12 Nonreactive evaporated was chosen as the overlayer for the first recipe comparison and is designed to prevent oxidation of the Ag during the subsequent reactive ALD or PVD deposition of the top layer. Previous work suggests that adheres well to Ag and may also offer the best adhesion surface for chemical vapor-based nucleation of the same material.13 Subsequently, sample A1 was removed from the PVD chamber and then loaded into the PEALD system where the sample was coated with 88 nm [i.e., “88 nm (ALD)”]. Meanwhile, sample A2 remained in the PVD chamber and was coated with 82 nm [i.e., “82 nm (PVD)”] by reactive e-beam IAD. The small difference in thickness (88 versus 82 nm) is not believed to be important and is consistent within the different measurement abilities. Samples B1 and B2 shown in Fig. 2 were designed to directly compare 105 nm barrier layers deposited by PEALD and reactive e-beam IAD with the incorporation of unique overlayers designed to enhance UV/blue reflectivity. Both samples utilized e-beam evaporation to concurrently deposit multiple layers, as displayed in Fig. 2, up to 36 nm . Prepared samples were subjected to environmental testing for 231 h () in an accelerated weathering environment described in Phillips et al.6 The samples were placed in a desiccator jar with wet KCl salt in the bottom to maintain relative humidity at , and the jar was placed in an oven maintained at 80°C. This aggressive environmental testing has been designed to push coating materials past failure and tends to yield at least some degree of degradation on even the best of coatings so that qualitative comparison can be made between these specific coating recipes. It should be noted that, in addition to the high temperature and humidity, there is an added component of salt ions in this environment. Samples are mounted vertically in the desiccator jar with only the bottom edge and corners in contact with the Delrin® mounting hardware in an effort to minimize potential condensation from contact with wet surfaces within the jar. Poststressing reflectivity measurements were made on “surviving” samples with enough remaining specular reflective surface area to fit within the spectrophotometer beam; any corroded regions lying in the spectrophotometer beam will scatter heavily and give a meaningless result. When a sample fails the environmental testing, it no longer functions as mirror to the eye, so it is not possible to obtain meaningful poststressing reflectivity due to the beam of the Cary 5000 spectrophotometer, which is much larger than the typical undamaged remaining area on a failed sample. However, the introduction of PEALD barrier layers has overcome the saturating over-aggressive effects of the environmental testing, and this study was able to include poststressing reflectivity curves for samples with a significant enough portion of remaining undamaged Ag which filled the fixed reflectivity measurement spot size. The implications of this new dimension of coating recipe experimentation will allow for more quantitative analysis in future work with PEALD layers. Samples A1 and B1 coated with PEALD were partially corroded in the environmental testing, however, the mirrors still functioned as specular reflectors to the naked eye, so poststressing reflectivity was measured. Fractional damaged areas of all four samples were estimated quantitatively using image processing software to identify corroded area ratios of each sample surface taken from side-by-side photography with uniform oblique lighting (Table 1). Table 1Fraction of damaged areas for samples A1 versus A2 and B1 versus B2.
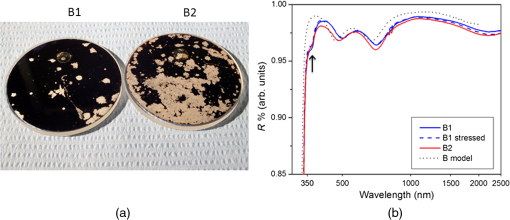
3.DiscussionReflectivity spectra are shown for all samples in Figs. 3 and 4 with a logarithmic -axis to emphasize visible/UV features. The resulting spectra reasonably match the reflectivity expected from coating models. In the case of the A1/A2 samples, this indicates the “antioxidation” overlayer was generally successful in preventing silver oxidation while transferring between chambers and during the ALD process. Fig. 3(a) Samples A1 and A2 after 231 h of environmental stress testing using a dark background and oblique lighting incident from the left, so dark areas indicate high specular reflectance while bright areas indicate scatter (corrosion). (b) Reflectivity is shown for A1 before and after the environmental testing; poststressing reflectivity could not be obtained from sample A2 due to the lack of measurable undamaged areas. The model reflectivity of the coating design is shown for comparison. The absorption feature near 370 nm (arrow) is caused by a surface plasmon resonance in the Ag; this feature is not predicted by the modeling software. 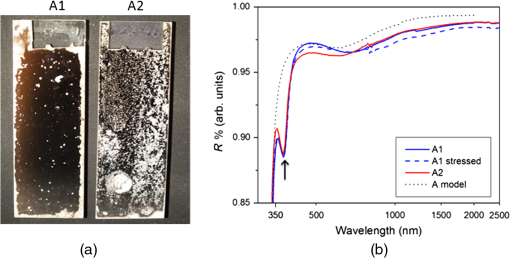 Comparison between samples A1 and A2, with reflectivity curves shown in Fig. 3, exhibits two spectra in reasonable agreement with the expected design reflectivity. The reflectivity model calculation does not account for absorption due to red-shifted surface plasmon resonance of metals with a changing dielectric function dependent on adjacent optical material,14 which is evident from the variation in reflectivity between and 400 nm. Small increases in surface root mean squared (RMS) roughness have been shown to induce some scattering and slightly decrease reflectivity.14 While the calculated reflectivity does not account for surface roughness, we expect minor surface roughness (RMS to 2 nm) for our mirror samples to explain the slight variation in reflectivity between simulated and experimental spectra. Sample A1 shows slightly higher reflectivity than A2 in the visible region. Since the only difference between samples A1 and A2 is the method of top barrier layer deposition, reactive e-beam IAD versus PEALD, this small difference in spectral response can be attributed to, for instance, the relative variation in film density of when comparing reactive e-beam IAD and PEALD. Alternatively, it may indicate that some ions penetrated the antioxidation barrier to produce some silver oxide in the PVD case of sample A2. Samples B1 and B2 were designed to repeat the comparison between top layers deposited by PEALD and reactive e-beam IAD, but with the application of a thicker overlayer stack to enhance UV/blue reflectivity. The 105 nm barrier layer is also the thickest of all samples in this study, which likely also contributes to the superb endurance of sample B1. Figure 4 shows the reasonably matched reflectivity curves of samples B1 and B2 with both curves varying slightly from simulated spectra based on similar model assumptions noted for samples A1 and A2. The slight increase in visible reflectivity of the sample B1 spectrum is attributed to the slightly lower optical density of PEALD-deposited as compared to the deposited by reactive e-beam IAD as previously mentioned when discussing samples A1 and A2. Figure 4 clearly shows that the UV/blue spectrum-boosting constructive interference stack design reflects more light in the measured spectral range than samples A1 and A2, and the PEALD barrier layer does not seem to impede this design. Sample B1 also shows the strongest relative endurance after the environmental testing. This observation can be correlated with the conclusion that thicker overlayers are likely to provide better corrosion protection. Sample B1 shows significantly less surface damage than B2 poststress testing, which is observable in Fig. 4. Figures 3 and 4 show environmental stress testing results with significantly less damage to PEALD-based samples A1 and B1 when compared to their reactive e-beam IAD counterparts, A2 and B2. The damaged area ratios of each sample are quantified in Table 1. Samples A1 and B1 show significantly less damaged Ag area and still yield specular reflection to the naked eye after environmental stress testing, therefore, reflectivity spectra were collected from these stressed samples. Partial corrosion of the barrier layers observed by the naked eye and reported in Figs. 3 and 4 indicate that improvements should still be made to the mirror stack design to ensure greater long-term endurance of the barrier layers. Visible results from Figs. 3 and 4 indicate damage to the reflective Ag surface within the mirror coatings, but further work is necessary to analyze any microstructural damage inflicted on the barrier film and surrounding layers during environmental testing. While measuring film thicknesses with spectroscopic ellipsometry before environmental testing, the refractive index of films grown by PEALD was found to be approximately 1.62 at 632 nm, which is slightly less than the typical value of 1.65 to 1.7 in films deposited by PVD techniques. Variations in film density and the resulting change in spectral response have been observed in similar experiments of oxide deposition comparing chemical vapor-based deposition with various PVD methods.15–18 Although higher film density may be intuitively desirable in corrosion barriers, resulting comparison between samples A1/A2 and B1/B2 shows that the slightly less dense films as deposited by PEALD create a more robust corrosion barrier. However, we attribute this enhanced robustness to the inherently conformal pinhole-free nature of the ALD monolayer growth process rather than the slightly lower film density. It is possible that the less dense films grown by PEALD sustain unseen damage such as increased porosity or hydrolyzed bonds19,20 which may require more sophisticated analysis techniques to observe. Schwinde et al.20 observe the hydrolyzation of similar barriers on Ag mirrors after exposure to moisture by scanning electron microscopy of barrier cross sections. The comparisons mentioned above were made to directly compare a single ALD versus PVD barrier layer with the remaining layers being identical. We have done several prior tests7,13,21 where either the “stacks” varied slightly, or the coatings were produced on different coating runs, leading to the concern that some other parameter might be influencing the results. However, in all of these tests, the ALD barrier layer samples consistently performed significantly better than the PVD counterparts, indicating the ALD barrier improved durability. The two tests reported in this work were designed to provide a direct comparison where only the top barrier layer differed, thus they have the most relevance. Therefore, future work will include observations and a more detailed analysis of various overlayer/underlayer materials correlating physical properties to their effect on overall mirror recipe performance in environmental testing. However, based on our previous reported observations, samples not included in this work, and the direct comparison between top barrier layer deposition method in this study, we qualitatively conclude that PEALD-based barrier layers offer a general advantage over reactive e-beam IAD layers in the environmental testing of mirror coatings. It should be noted that Schwinde et al.20 included some ALD samples in their study of protected-Ag coatings and reported no improvement in coating durability over PVD samples. The reason for this is unclear. It is possible their PVD process was “cleaner” and thus had fewer pinholes than our samples, which would implicate pinholes as the dominant cause leading to degradation. It is also possible that some other variables in the coating process were responsible for the different results. We note that their environmental stressing involved condensing water onto the surface of their samples, which may have accelerated hydrolyzation of the aluminum oxide. In any event, we have found consistently better performance with ALD in multiple tests. We believe our cleaning and coating processes are typical for astronomical mirrors, and the environmental stressing with humid air is more representative of typical conditions for the mirrors, so our results may have more relevance to practical coatings for astronomical mirrors. However, the conflicting findings indicate more work is needed to fully understand all the parameters involved. 4.ConclusionHigh-reflectivity silver mirrors with two corrosion barrier recipes have been fabricated using e-beam IAD and PEALD. Mirrors were environmentally stress tested in an accelerated environmental aging process using high temperature/high humidity conditions at 80°C and humidity for . Reflectivity was measured prestress and poststress testing, and visible surface damage was assessed in a comparison between deposition methods for top barrier layers of . Our pilot study of PEALD-based barriers has not yielded a perfect silver mirror coating; however, we have strong evidence that the ALD process does have promise as protection against tarnish and corrosion in addition to its inherently excellent optical properties. Environmental testing shows significantly higher endurance for mirrors coated with deposited by PEALD when compared with similar coatings deposited by reactive e-beam IAD. Further work on deposition process parameters and material layer selection will be integral to finding an optimized mirror coating recipe, and more precise control and analysis of environmental stressing and poststress measurements will yield better understanding of the corrosion mechanisms observable on silver mirrors. AcknowledgmentsThe UCO coating chamber has been made possible through support by the National Science Foundation under Grant No. 1005506. We would like to thank HP Labs of Palo Alto, California, for their assistance with PEALD deposition. ReferencesM. R. Jacobson et al.,
“Development of silver coating options for the Gemini 8-m telescopes project,”
Proc. SPIE, 3352 477
–502
(1998). http://dx.doi.org/10.1117/12.319269 PSISDG 0277-786X Google Scholar
M. Boccas et al.,
“Coating the 8-m Gemini telescopes with protected silver,”
Proc. SPIE, 5494 239
(2004). http://dx.doi.org/10.1117/12.548809 PSISDG 0277-786X Google Scholar
T. Vucina et al.,
“Gemini primary mirror in-situ wash,”
Proc. SPIE, 7012 70122Q
(2008). http://dx.doi.org/10.1117/12.788388 PSISDG 0277-786X Google Scholar
A. C. Phillips et al.,
“Progress toward high-performance reflective and anti-reflection coatings for astronomical optics,”
Proc. SPIE, 7018 70185A
(2008). http://dx.doi.org/10.1117/12.789862 PSISDG 0277-786X Google Scholar
A. C. Phillips et al.,
“Progress toward high-performance astronomical coatings,”
Proc. SPIE, 7739 77393Y
(2010). http://dx.doi.org/10.1117/12.856499 PSISDG 0277-786X Google Scholar
A. C. Phillips et al.,
“Progress in UCO’s search for silver-based telescope mirror coatings,”
Proc. SPIE, 8450 84502G
(2012). http://dx.doi.org/10.1117/12.925502 PSISDG 0277-786X Google Scholar
A. C. Phillips et al.,
“Progress and new techniques for protected-silver coatings,”
Proc. SPIE, 9151 91511B
(2014). http://dx.doi.org/10.1117/12.2055706 PSISDG 0277-786X Google Scholar
J. D. Barrie et al.,
“Control of stress in protected silver mirrors prepared by plasma beam sputtering,”
Appl. Opt., 50
(9), C135
–C140
(2011). http://dx.doi.org/10.1364/AO.50.00C135 APOPAI 0003-6935 Google Scholar
C. T. Chu, P. D. Fuqua and J. D. Barrie,
“Corrosion characterization of durable silver coatings by electrochemical impedance spectroscopy and accelerated environmental testing,”
Appl. Opt., 45
(7), 1583
–1593
(2006). http://dx.doi.org/10.1364/AO.45.001583 APOPAI 0003-6935 Google Scholar
P. J. Jobst et al.,
“Optical properties of unprotected and protected sputtered silver films: surface morphology vs. UV/VIS reflectance,”
Adv. Opt. Technol., 3
(1), 91
–102
(2014). http://dx.doi.org/10.1515/aot-2013-0052 Google Scholar
A. C. Phillips et al.,
“Fluoride damage to substrates during stripping of mirrors,”
Proc. SPIE, 9151 91515I
(2014). http://dx.doi.org/10.1117/12.2056887 PSISDG 0277-786X Google Scholar
H. von Seefeld et al.,
“Investigation of titanium—nitride layers for solar-cell contacts,”
IEEE Trans. Electron Devices, 27
(4), 873
–876
(1980). http://dx.doi.org/10.1109/T-ED.1980.19949 Google Scholar
D. M. Fryauf, A. C. Phillips and N. P. Kobayashi,
“Moisture barrier and chemical corrosion protection of silver-based telescope mirrors using aluminum oxide films by plasma-enhanced atomic layer deposition,”
Proc. SPIE, 8820 88200Y
(2013). http://dx.doi.org/10.1117/12.2023826 PSISDG 0277-786X Google Scholar
H. E. Bennett, J. L. Stanford,
“Structure-related optical characteristics of thin metallic films in the visible and ultraviolet,”
in Standardization in Spectrophotometry and Luminescence Measurements: Proc. of a Workshop Seminar Held at the National Bureau of Standards,
133
–148
(1976). Google Scholar
P. J. Martin,
“Ion-based methods for optical thin film deposition,”
J. Mater. Sci., 21
(1), 1
–25
(1986). http://dx.doi.org/10.1007/BF01144693 JMTSAS 0022-2461 Google Scholar
M. D. Groner et al.,
“Low-temperature atomic layer deposition,”
Chem. Mater., 16
(4), 639
–645
(2004). http://dx.doi.org/10.1021/cm0304546 CMATEX 0897-4756 Google Scholar
V. Cimalla et al.,
“Densification of thin aluminum oxide films by thermal treatments,”
Mater. Soc. Appl., 5 628
–638
(2014). http://dx.doi.org/10.4236/msa.2014.58065 Google Scholar
C. Cibert et al.,
“Properties of aluminum oxide thin films deposited by pulsed laser deposition and plasma enhanced chemical vapor deposition,”
Thin Solid Films, 516
(6), 1290
–1296
(2008). http://dx.doi.org/10.1016/j.tsf.2007.05.064 THSFAP 0040-6090 Google Scholar
K. Wefers and M. Chanakya, Oxides and Hydroxides of Aluminum, 1987). Google Scholar
S. Schwinde et al.,
“Description of particle induced damage on protected silver coatings,”
Appl. Opt., 54 4966
–4971
(2015). http://dx.doi.org/10.1364/AO.54.004966 APOPAI 0003-6935 Google Scholar
A. C. Phillips, D. M. Fryauf and N. P. Kobayashi, Google Scholar
BiographyDavid M. Fryauf is a research student pursuing his PhD in electrical engineering at the University of California Santa Cruz. He received his BS in electrical engineering from the University of Arkansas, Fayetteville. His research interests are in thin film deposition and optical/electrical material properties for applications, including thermoelectric power generation, memristor devices, anti-reflection coatings, and corrosion barriers for optical components. Nobuhiko P. Kobayashi is a professor of the Basking School of Engineering at University of California, Santa Cruz (UCSC). He conducts research encompassing low-dimensional materials and devices. Prior to UCSC, Kobayashi was at various places, including Hewlett-Packard Laboratories and Lawrence Livermore National Laboratory, developing such materials and devices as compound semiconductors for high-speed communication and diagnosis systems and resistive switches for future computers. He earned a PhD degree in material science at the University of Southern California in 1998. |